Sani-Tech® Ultra The Next Generation Tubing
Sani-Tech® Ultra represents the next generation in ultra-pure platinum-cured silicone
tubing. Quite simply, it’s a better product. Features like ultra-low extractables and longer
pump life were engineered into Ultra by the experienced materials scientists of Saint
Gobain Performance Plastics to meet the stringent requirements of the biopharmaceutical
market.
Applications
Sterile fill and transfers
Drug delivery
Cell harvest
Media processing
Sanitary peristaltic pump tubing
Production filtration and fermentation
Bioreactor process lines
Characteristics
Fluid integrity is vitally important in many biopharmaceutical applications. Sani-Tech® Ultra formulations are manufactured and packaged in a certified clean room from the finest grade of silicone materials. Ultra-low TOCs ensure that contamination by extractables is kept to an absolute minimum. Saint-Gobain Performance Plastics’ silicone tubing is known for its superior pump life — and Sani-Tech® Ultra is no exception. In all our multiple production sites (US, France, and India) our tubing are produced and double bag packaged in an ISO 7 clean room.
Biocompatibility
Sani-Tech® Ultra is manufactured from the finest grade of silicone materials and is fully characterized, validated and tested to a variety of specifications including USP Class VI, ISO 10993, and European Pharmacopoeia 3.1.9. For additional compliance data visit www. biopharm.saint-gobain.com to download Validation Guide and Regulatory Information Overview (RIO) or contact our customer service department. Sani-Tech® Ultra has a Drug Master File Type II with the U.S. Food and Drug Administration.
Features / Benefits
Two hardnesses available, 50 and 65 shore
Mulitple manufacturing sites: US, France, and India
Biopharmaceutical grade
Ultra-low extractables
Excellent flexibility
Laser etched for lot traceability
Superior pump life
Fully autoclavable and sterilizable
Non-yellowing formulation
Temperature range from -80°F (-62°C) to 500°F (260°C)
Meets USP Class VI, EP 3.1.9 and ISO 10993
Available in 25-, 50- and 100-foot coils
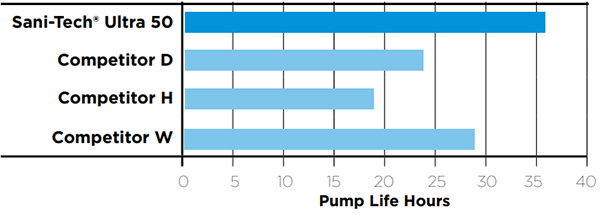
Comparative Peristaltic Pump Tubing Life
The table below depicts hours until failure of 1/4" ID x 3/8" OD tubing. In each case, a 3-roller pump head was utilized operating at 600 RPM at room temperature (73°F)
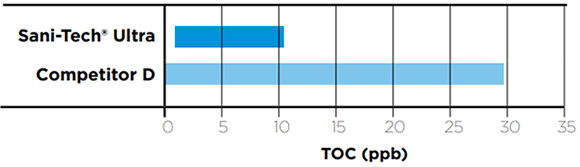
Comparative Extractables Concentrations
The table below illustrates the TOC concentrations of post autoclaved tubing samples. In each case the samples were subjected to a DI water flush and the effluent was analyzed.
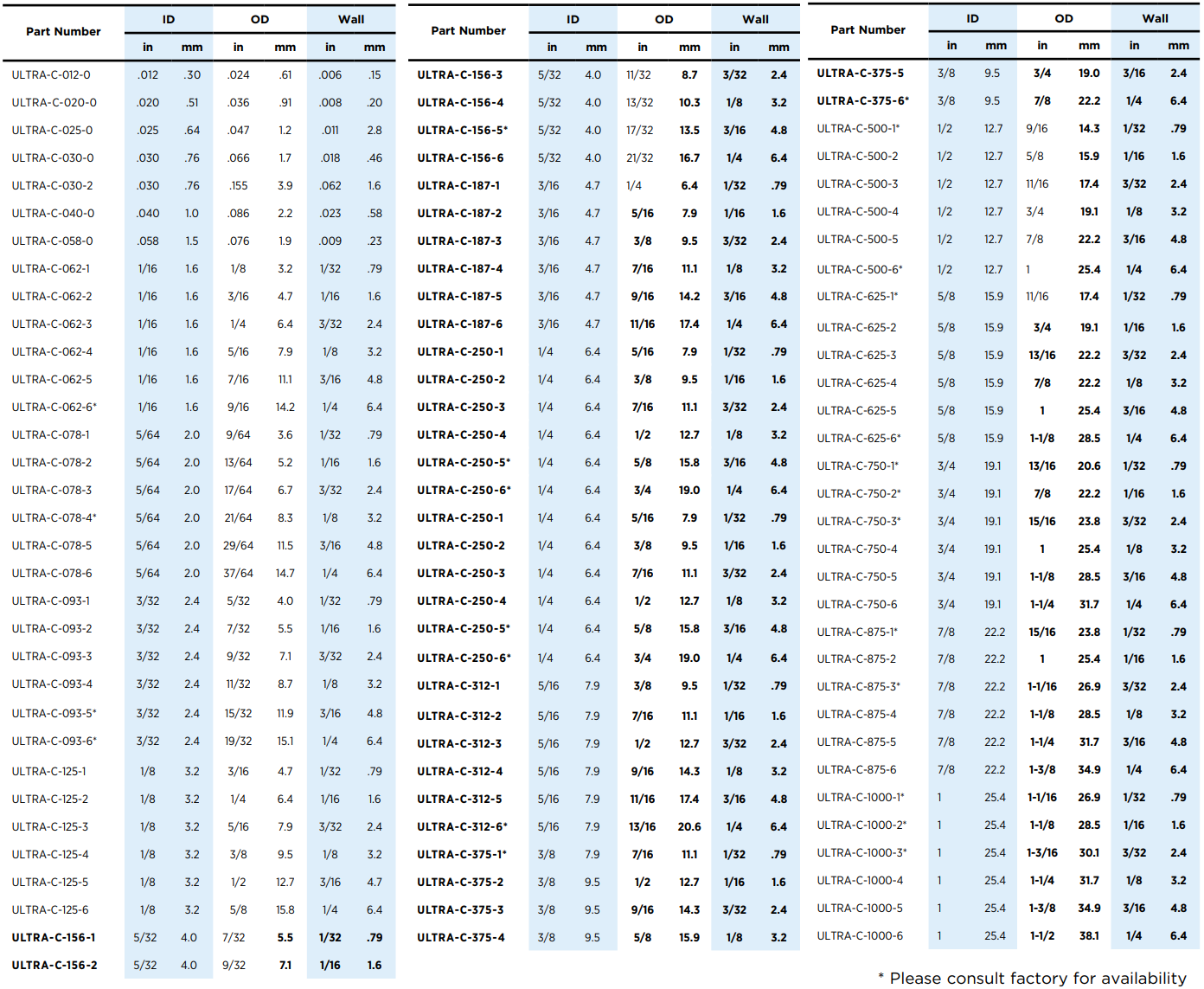
Sani-Tech® Ultra Tubing Available Sizes
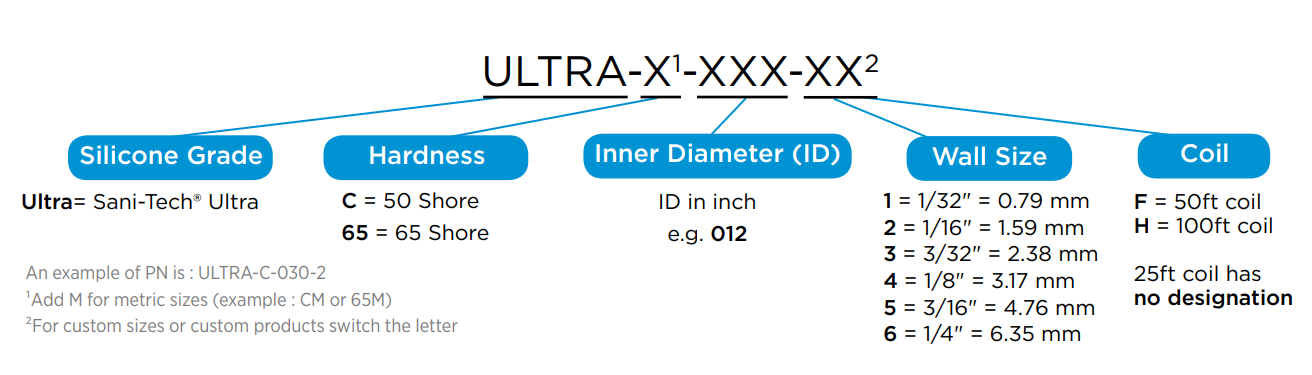
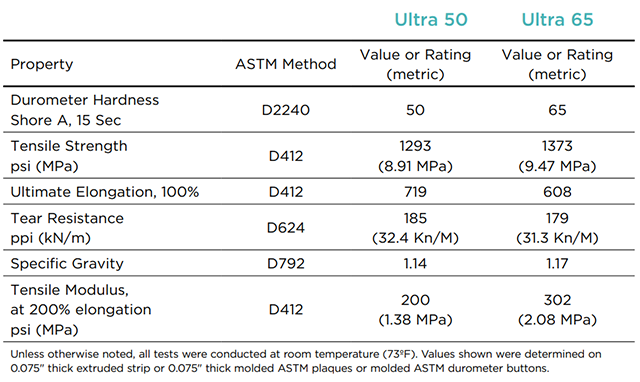
Typical Physical Properties
Characterization
The bio-compatibility of Sani-Tech® Ultra platinum-cured silicone tubing, manufactured with Sani-Tech® silicone, has been tested and complies with the parameters set forth in the following test protocols:
USP Class VI (88) biological reactivity, in vivo - Intracutaneous test, Systemic injection test, Implantation test
USP 87 biological reactivity, in vitro - L929 MEM elution, AGAR diffusion
USP 381 elastomeric closure for injection
USP 85 endotoxin
European Pharmacopoeia 3.1.9
USP limits for total organic carbons
Gamma irradiation
Infrared analysis
ISO 10993
NOTE: Sani-Tech® Ultra tubing will not deteriorate with repeated autoclaving. This method of sterilization is strongly recommended. Sani-Tech® Ultra silicones should not be considered for consistent steam applications. WARNING: Do not use Sani-Tech® Ultra silicone tubing in hot oil or acid applications.
Sani-Tech® Ultra Options
Custom laylines
Gamma irradiated
Bulk spool packaging
Custom sizes available
Available in custom lengths and durometers
Sterilization Methods
Autoclavable
Gamma irradiation
Up to 5.0 Mrad (50 Kilogray)
Gas - ethylene oxide

