Good to know 2
What are leachables and extractables?
-
Extractables - Compounds that can be extracted from elastomeric, plastic components or coatings of the container and closure system when in the presence of an appropriate solvent(s).
-
Leachables - Compounds that leach from elastomeric, plastic components or coatings of the container and closure system as a result of direct contact with the formulation.
-
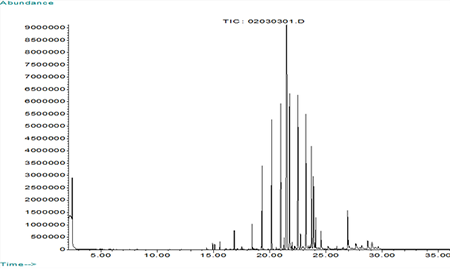
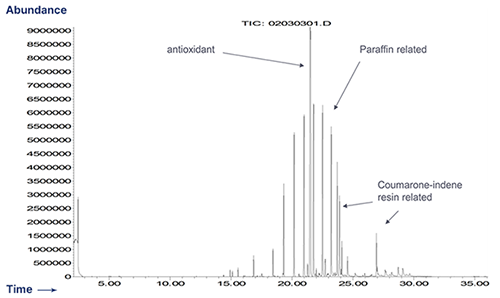
GC/MS Extractables Profile of an Elastomer
-
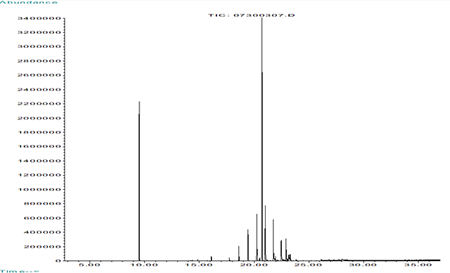
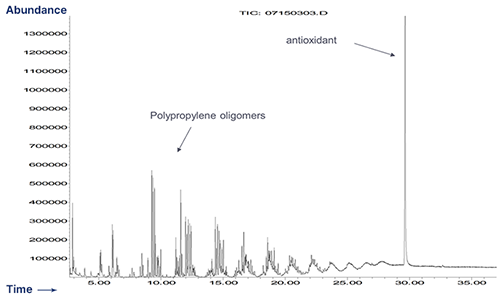
GC/MS Extractables Profile of Polypropylene
-
Structure and Fragmentation of Irganox 1076 in Positive Ion APCI (MS/MS)
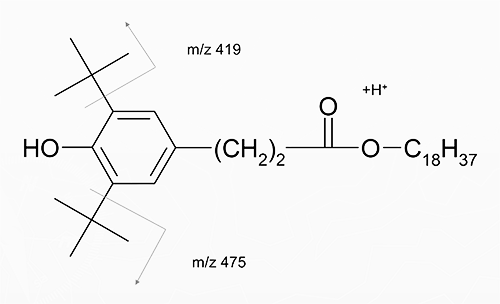
-
MS/MS Spectrum of Irganox 1076
(ESI+; products of m/z 531)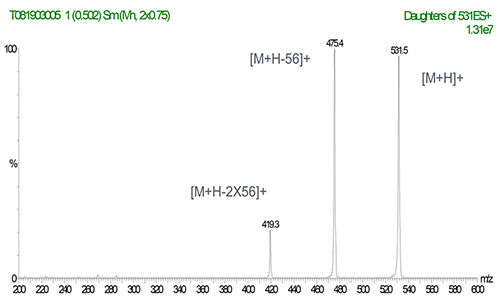
-
Structure and Fragmentation of Tetramethylthiuram Disulfide in Positive Ion APCI (MS/MS)
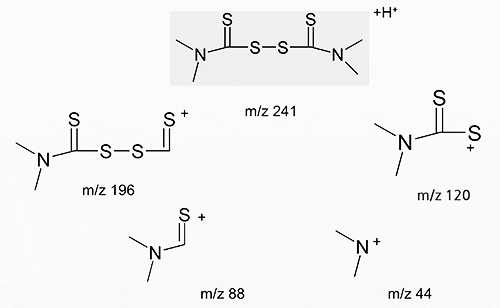
-
MS/MS Spectrum of Tetramethylthiuram Disulfide
(ESI+; products of m/z 241)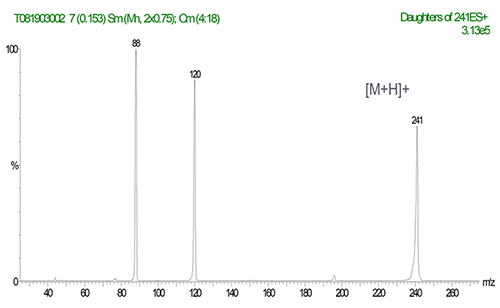
-
Rubber Formulation A (Sulfur Cured)
Ingredient % CALCINED CLAY 8.96 BLANC FIXE (barium sulfate) 25.80 CREPE 38.22 BROWN SUB MB 16.84 1722 MB 2.11 ZINC OXIDE 4.04 2,2' METHYLENE-BIS (6-TERTIARY BUTYL-4-ETHYL PHENOL) 0.56 COUMARONE-INDENE RESIN 1.12 PARAFFIN 1.12 TETRAMETHYLTHIURAM MONOSULFIDE 0.11 ZINC 2-MERCAPTOBENZOTHIAZOLE 0.29 SULFUR 0.84 -
What do we know?
Carbon black is a known source of PAHs and has also been shown to be involved in N-nitrosamine formation in rubber ("special cases").
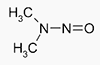
Thiurams are known precursors of N-nitrosamines.
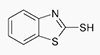
2-Mercaptobenzothiazole is a known "special case".
Paraffin and Coumarone-indene resin are natural product materials and are likely complex mixtures of related structures.
Individual additives are likely GC-able.
-
Sulfur Cured Rubber - Extractables Profile by GC/MS
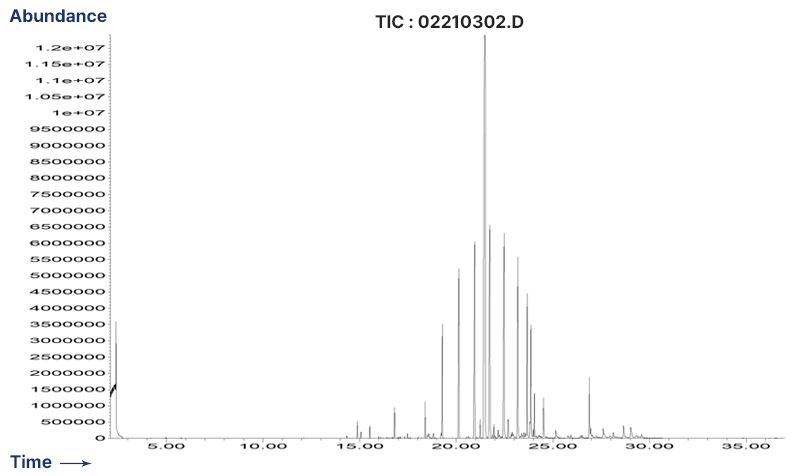
-
Rubber Formulation B (Peroxide Cured)
Ingredient % IMSIL A25 (silicone dioxide) 24.01 MISTRON CYPRUBOND (magnesium silicate) 19.21 BROMOBUTYL 2030 38.42 VISTALON 404 (ethylene propylene copolymer) 9.61 WHITE OIL 2 1.44 420 BLUE MB 0.12 TITANIUM DIOXIDE 1.68 PARAFFIN 0.96 MAGNESIUM OXIDE 0.60 STEARIC ACID 0.48 POLYETHYLENE WAX 1.44 P-800 2.03 CONTENTS OF 420 BLUE MB
SBR-3
PIGMENT BLUE 15
ANOX 9
CONTENTS OF P-800
2,5 DI METHYL-2, 5-DI (T-BUTYL PEROXIDE) HEXANE 68.00 PRECIPITATED SILICA 32.00 -
What do we know?
Registry Number: 78-63-7

Formula: C16 H34 04
CA Index Name:
Peroxide, (1,1,4,4-tetramethyl-1,4-butanediyl)bis[(1,1-dimethylethyl) (9CI)Other Names:
Peroxide, (1,1,4,4-tetramethyltetramethylene)bis(tert-butyl (6CI,8CI); (1,1,4,4- Tetramethyltetramethylene)bis(tert-butyl peroxide); 101XL; 2,5-Bis(tert-butyldioxy)-2,5-dimethylhexane; 2,5-Bis(tert- butylperoxy)-2,5-dimethylhexane; 2,5-Di(t-butylperoxy)-2,5-dimethylhexane; 2,5-Di(tert-butylperoxy)-2,5- dimethylhexane; 2,5-Di-tert-butyl-2,5-dimethylhexyl peroxide; 2,5-Dimethyl-2,5-bis(tert-butyldioxy)hexane; 2,5- Dimethyl-2,5-bis(tert-butylperoxy)hexane; 2,5-Dimethyl-2,5-di(t-butylperoxy)hexane; 2,5-Dimethyl-2,5-di(tert- butylperoxy)hexane; 2,5-Dimethyl-di(tert-butyl)peroxyhexane; 2,5-Dimethylhexane-2,5-di-tert-butylperoxide; 2,5- Methyl-2,5-di(tert-butylperoxy)hexane; 25B40; AD; AD 40C; ÁPO; APO 40S; C 15; C 15 (peroxide); C8; C8 (vulcanizer); C 8A; CR 05; CT 8; CT 8 (crosslinking agent); HC 4; HC 4 (peroxide); Interox DHBP; Interox DHBP 45IC/G; Kayahexa AD; Kayahexa AD 40; Kayahexa AD 40C; L 101; LX 101; Link-Cup DBPH; Luperco 101X45; Luperco 101XL; Luperox 101; Luperox 101XL; Luperox 101XL45; Lupersol 101; Lupersol 101XL; Lupersol L 101; NSC 38203; Perhexa 2.5B; Perhexa 2.5B40; Perhexa 25B; Perhexa 25B40; RC 4; RC 4 (peroxide); RC 450P; RC 8; RPZ 101; Sanperox APÓ; TC 8; TC 8 (catalyst); Trigonox 101; Trigonox 101-40D; Trigonox 101-40MD-GR; Trigonox 101-50; Trigonox 101E10; Trigonox 101E5; Trigonox XQ 8; Varox; Varox 50; Varox DBPH; Varox DBPH 50; Varox Liquid; Yinox 1012261 references in chemistry database (7 related to analytical studies)
-
Peroxide Cured Rubber - Extractables Profile by GC/MS
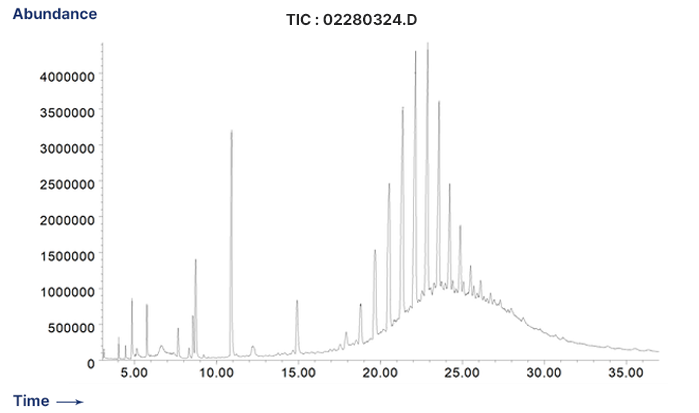
-
Polypropylene Formulation
Ingredient wt % ► Primary Stabilizers
Tetrakis (methylene(3,5-di-t-butyl- 4-hydroxyhydrocinnamate)) methane Irganox 1010 (Ciba) Anox 20 (Great Lakes) 0.08 wt% ► Secondary Stabilizers
Bis(2,4-di-t-butylphenyl)pentaerythritol diphosphite Ultranox 626 (GE) 0.05 wt% ► Corrosion Inhibitors
Calcium Stearate 114-50 (Ferro) 0.03 -0.4 wt% ► Antistatic
Vegetable oil derived
90% alpha monoglycerides (soybean) Pationic 901 (Patco)
Dimodan HS-KA (Danisco)0.3 wt% ► Nucleating Agents
3,4-dimethyl dibenzylidene sorbitol
Millad 3988 (Milliken)0.2 wt% -
What do we know?
Polypropylene is known to contain many soluble oligomers.
Individual additives will likely require analysis by HPLC based methods.
Individual additives could be both chemically complex and have complex degradation chemistries.
No reason to suspect the presence of "special cases"
-
Polypropylene - Extractables Profile by LC/UV/MS
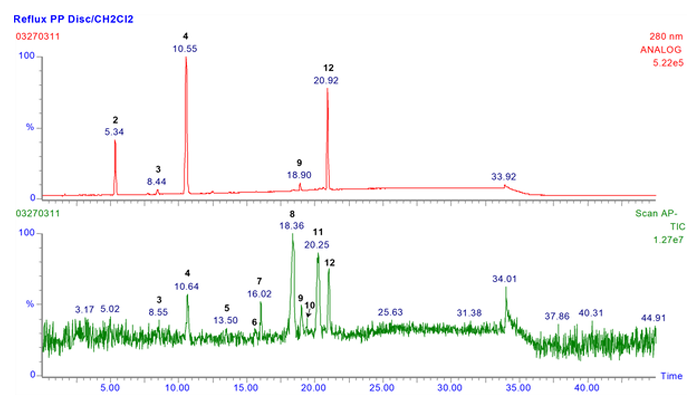
Polypropylene - Extractables Profile by GC/MS
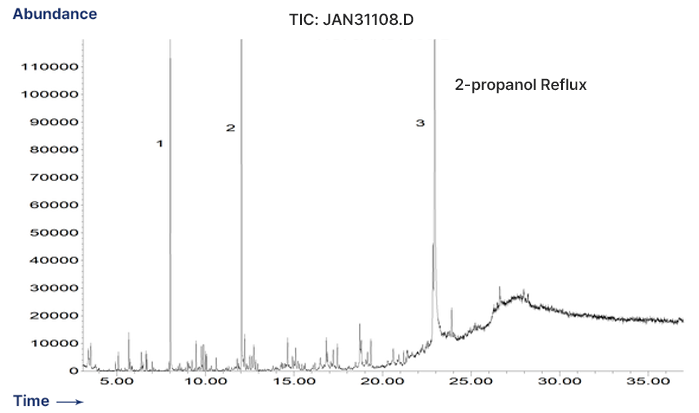
-

Best Practices for OINDP Pharmaceutical Development Programs Leachables and Extractables
VII. Special Case Compound Classes
PQRI Leachables & Extractables Working Group
PQRI Training Course September 20-21, 2006
Washington, DC
-
"Special Cases"
PAHs - Polyaromatic Hydrocarbons
Also referred to as PNAS (Polynuclear Aromatics)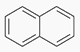
N-Nitrosamines
2-Mercaptobenzothiazole
-
PAHs/PNAs as Leachables in OINDP
Historically, the primary source of PNAS is carbon black which is used as a filler in certain types of rubber (mostly sulfur cured).
There is some potential for other PNA sources (e.g.naphthalene contamination).
Some PNAs are known or suspect cancer causing agents (e.g. benzo(a)pyrene). FDA interest in MDIs traces back to the late 1980s.
Levels of PNAs in MDIs which employ "black rubber" seals are typically on the order of ng to low μg/canister.
The FDA historically requires that all elastomers in MDIs be evaluated and controlled for PNAS.
Analytical methods typically involve GC/MS.
-
PNAS Typically Analyzed and Controlled
(EPA Method 610 list)Naphthalene
Acenaphthylene
Acenaphthene
Fluorene
Phenanthrene
Anthracene
Fluoranthene
Pyrene
Benzo(a)anthracene
Chrysene
Benzo(b)fluoranthene
Benzo(k)fluoranthene
Benzo(e)pyrene
Benzo(a)pyrene
Indeno(123-cd)pyrene
Dibenzo(ah)anthracene
Benzo(ghi)perylene
-
Threshold Concept

-
Concept: AET and Chromatography
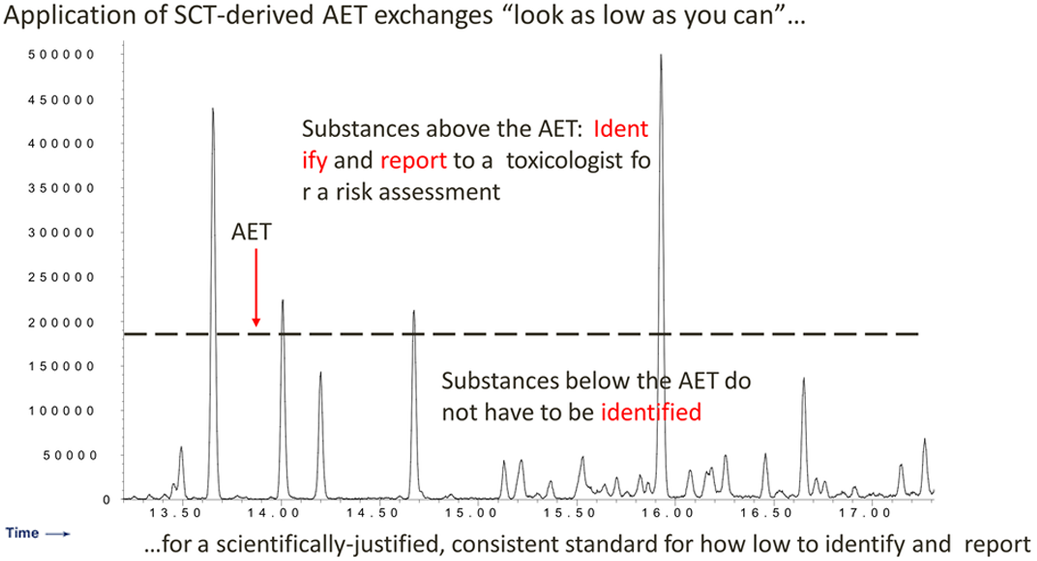
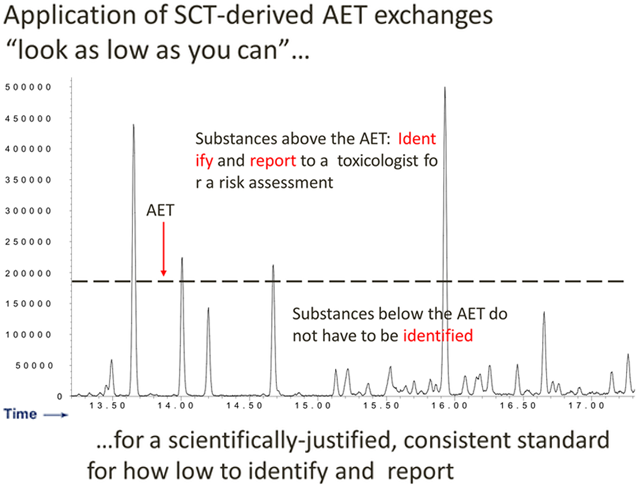
-
PDP Identification Threshold: SCT Derived
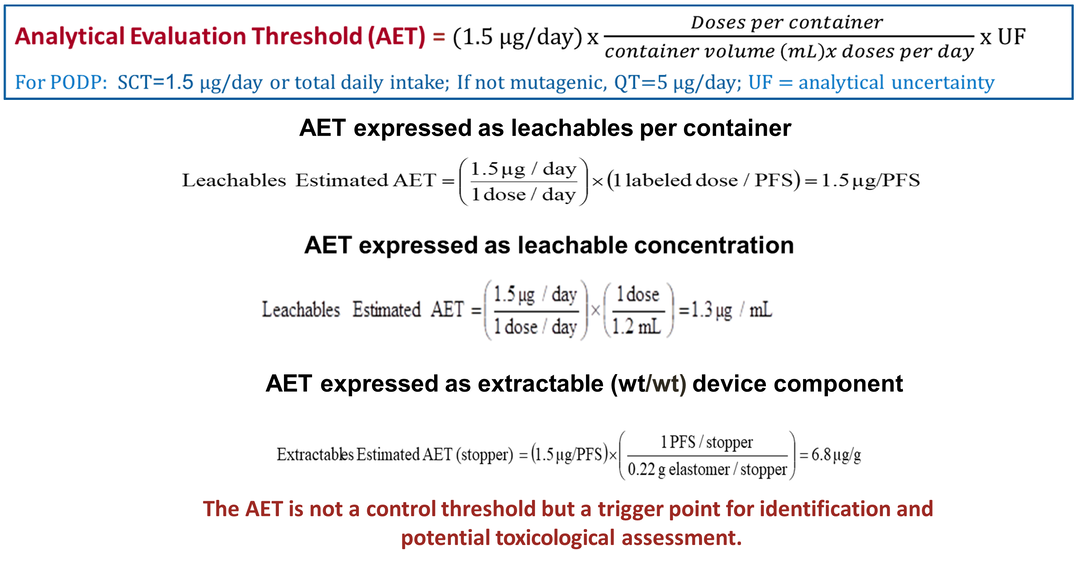
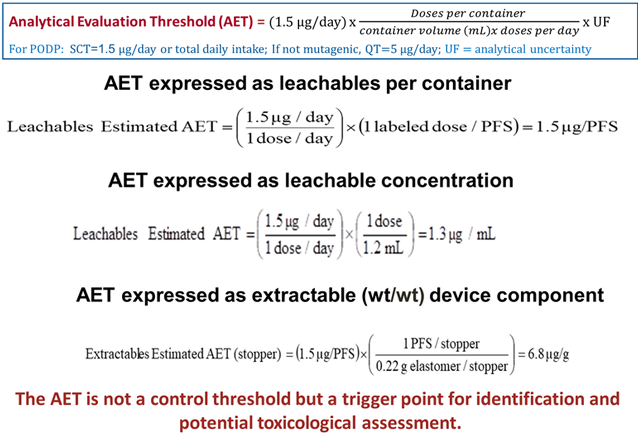
-
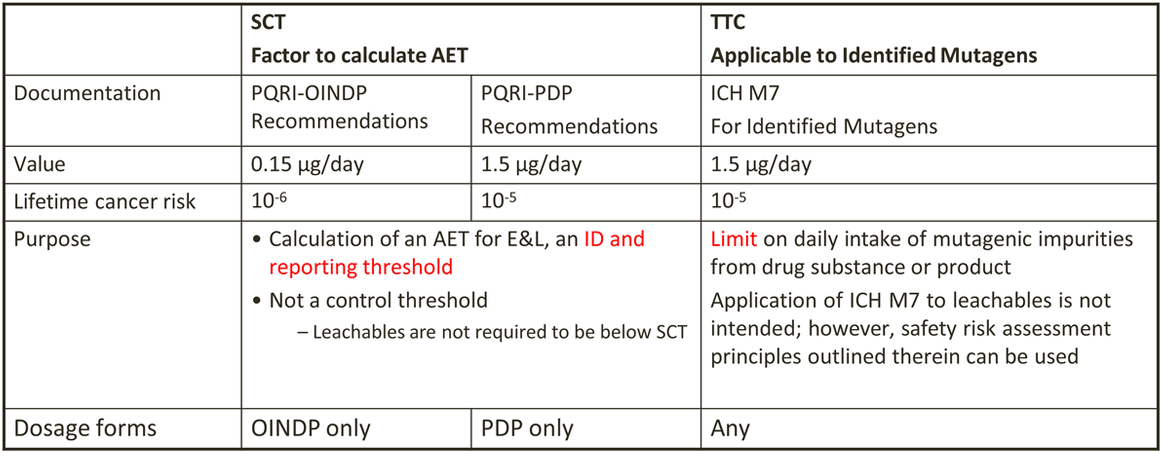
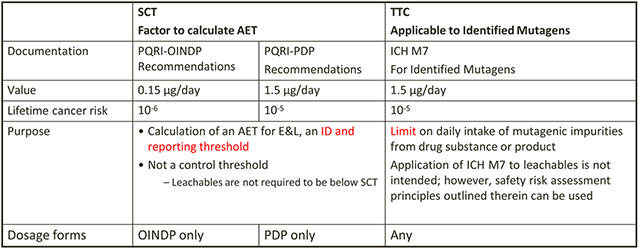
SCT vs TTC and ICH M7
-
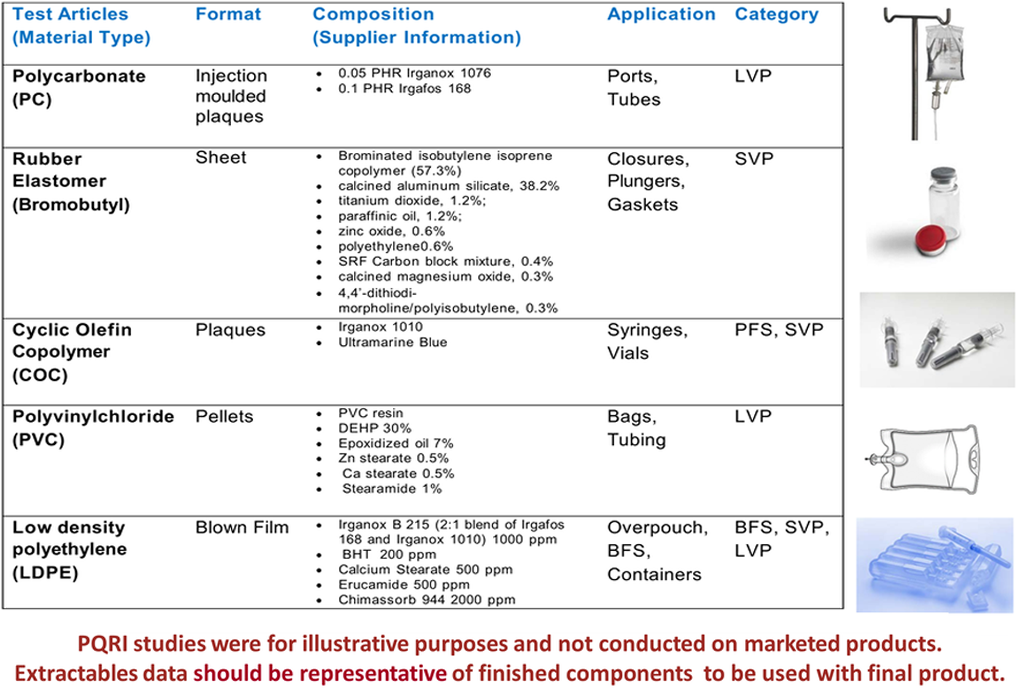
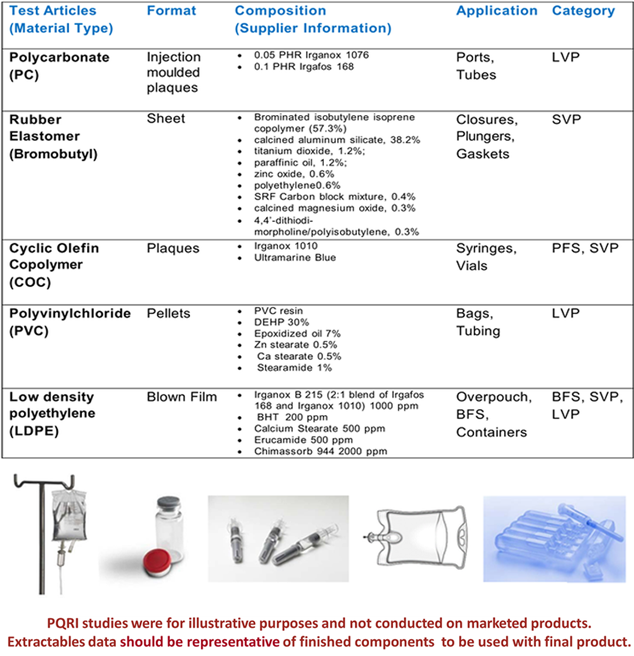
PDP Extractables Material of Construction
-
Extractables and Leachable Studies
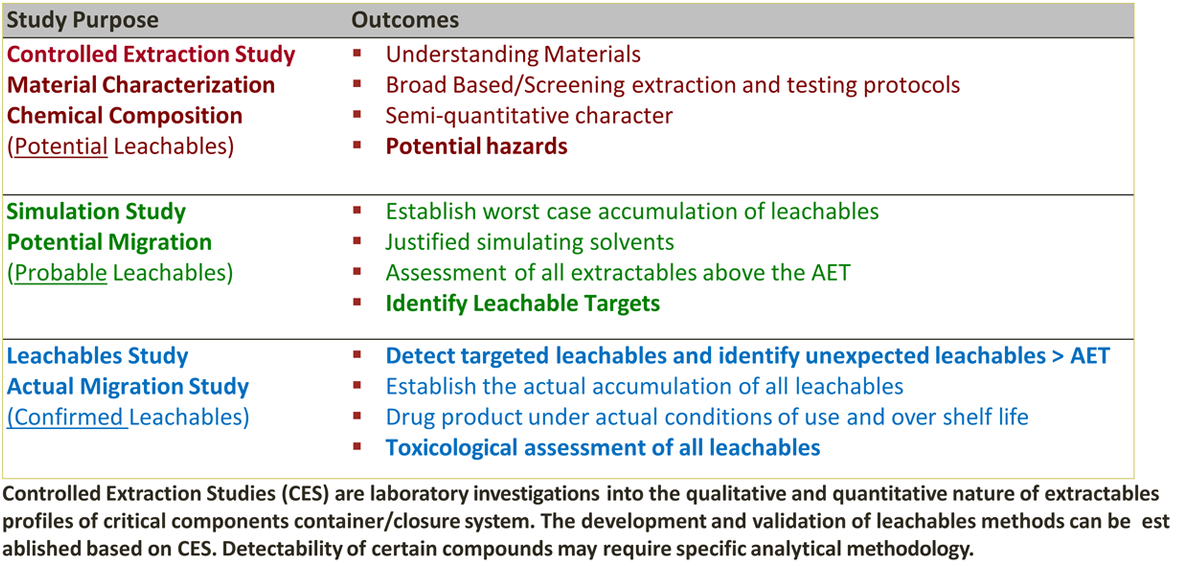
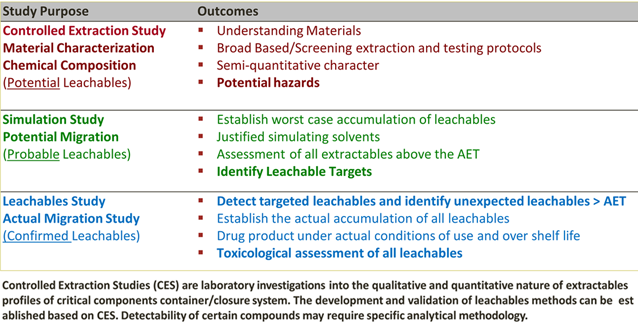
-
Holostic Leachable Risks
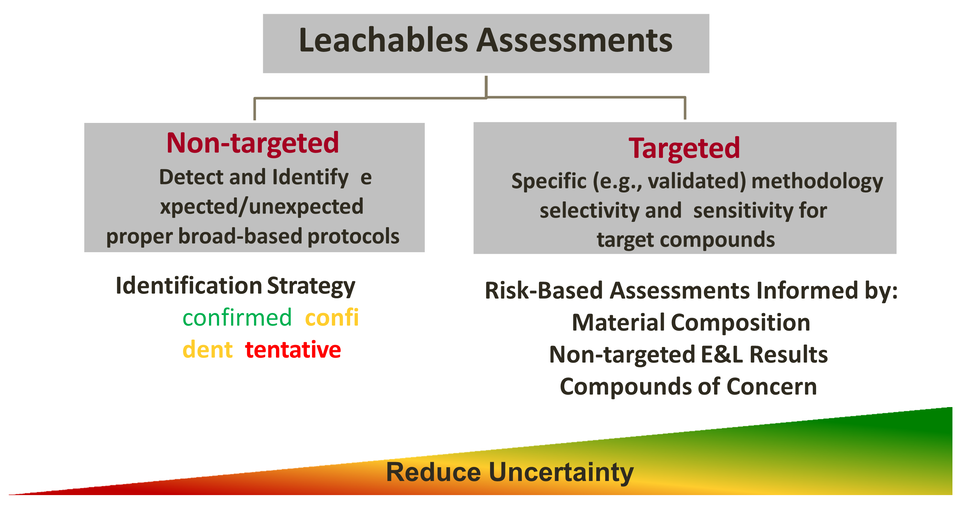
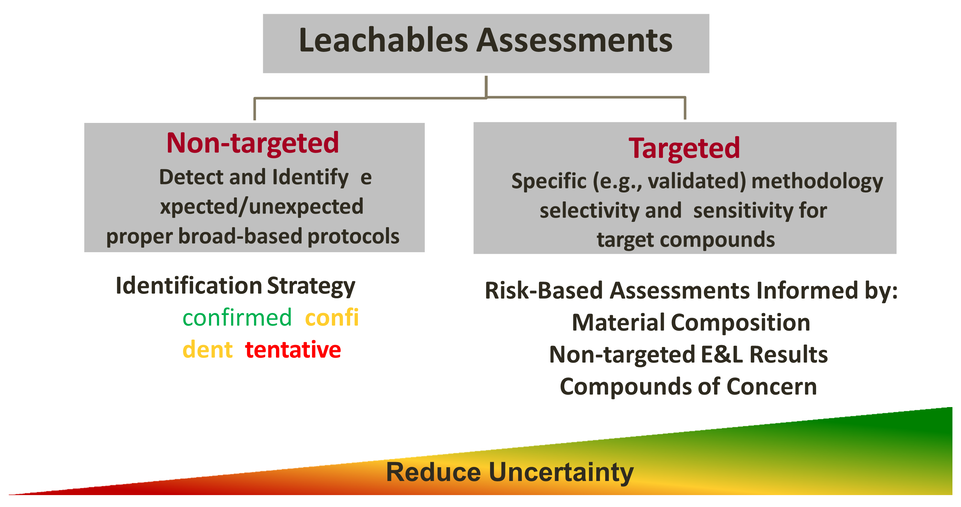
-
Analytical Methods Typically Employed
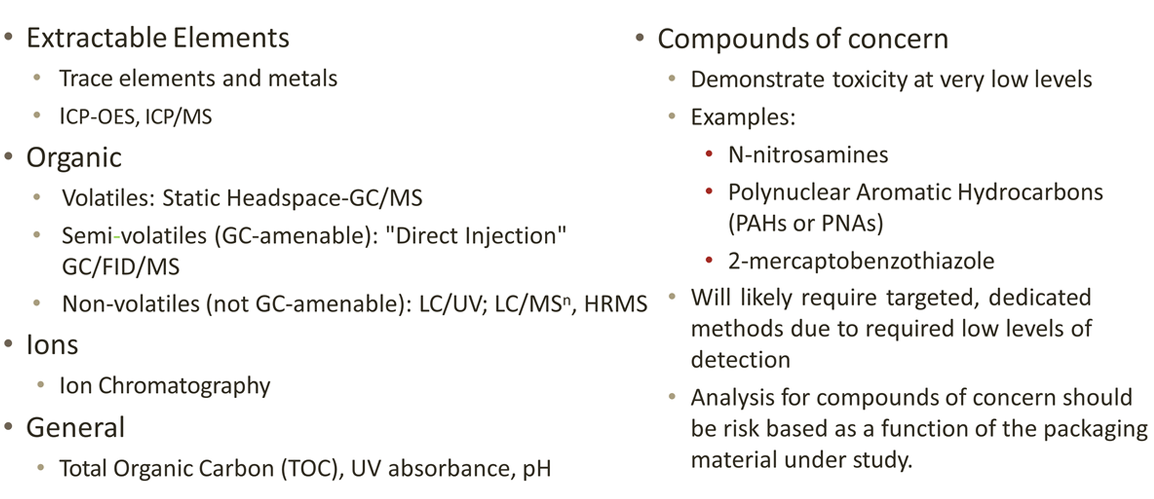

Summary of PDP Recommendations
-
1. An SCT approach can be applied to L&E qualification in parenteral drug products
1.5 μg/day for organic leachable can be used to calculate an AET for identification purposes.
An identification threshold lower than 1.5 μg/day may be warranted for certain classes of compounds
2. If identified leachables are non- mutagenic a Qualification Threshold (QT) at ≤5 μg/day is appropriate
A non- mutagenic leachable which has potential for irritation or sensitization should be controlled at ≤5 μg/day
3. Extractables assessments for PDP should be considered for appropriate materials of construction, finished components, and complete packaging systems (i.e., container closure systems).
4. Extractables assessments for PDP packaging systems should include aqueous-based extraction solvents with consideration of extraction pH, organic solvent content, and other appropriate extraction conditions
e.g., extraction time, extraction temperature, extraction technique, and sample-to-solvent ratio.
Extractable studies for CCS used with complex drug products (e.g., emulsion or polymeric additives) should c onsider appropriate solvent propensity to establish the extractable profile to guide optimization of non- targeted assessment methods for placebo or leachables
-
5. Where appropriate, L&E assessments, for PDP and their packaging systems should consider the possibility of migration across packaging barriers.
i.e., drug product labels, adhesives, inks, etc.
6. Certain PDP (e.g., large volume parenterals), will have analytically challenging AETS, and a "Simulation Study," could supplement and guide subsequent drug product leachables studies.
Simulation results can establish an extractables profile to inform on a probable leachables profile of the packaged drug product that the study simulates. Use of a simulation study would need to be appropriately justified.
7. Biologics have unique considerations compared to chemically synthesized drug products. Considerations for comprehensive risk assessments should include biologic activity, efficacy and safety related to the following
Leachable interactions affecting product quality attributes, i.e., degradation, oxidation, chemical modification, immune adjuvant activity.
Aspects of material compatibility, surface characteristics, organic/inorganic alert compounds.
Individual components and system interfaces, performance and functionality.
Leachables assessment performed on the product under stress conditions, and under real-time storage.
Due to the increasing complexity of pharmaceutical products and container closure systems, justifications for the AET, extraction conditions, extraction solvents and analysis should be discussed early with the Regulatory Agency/Division.
FAQ
-
QWhat are expectations around ophthalmic drug products with respect to SCT?A
In an effort to standardize E&L practices across drug products, ODP were originally in scope for the Product Quality Research Institute Leachables and Extractables Working Group for Parenteral and Ophthalmic Drug Products (PQRI-PODP). However, the working group realized over time that parenteral and ophthalmic drug products are sufficiently different that they cannot be readily treated in the same manner. For i njectable drug products, an SCT could be generated based on similar principles originally used by the OINDP working group such as systemic toxicology. Typical ODP, on the other hand, are dosed topically in sm all aliquots directly to the eye. Currently there is not a sufficient database developed on all the relevant toxicity endpoints to allow the working group to recommend specific safety thresholds (i.e., sensitization , ocular irritation) for ODP at this time. Thus, the hypothesis that threshold principles could be extrapolated from OINDP to ophthalmic solutions and suspensions lacked sufficient scientific support to develop a recommendation.
-
QCan simulation / extraction studies be done in lieu of leachables?A
Citing examples where leachables were observed in drug product that were either unique from or present at higher levels than what was observed in extraction studies, regulators are cautious about assessments that rely solely on extraction (or simulation) studies without a corresponding leachable study. As a result, sponsors should generally plan to perform confirmatory leachable testing with drug product fol lowing extraction studies. If a sponsor feels that there is a strong scientific basis to forego leachable testing based on extraction results, consultation with regulatory authorities is recommended.
-
QCan we use supplier data to support E&L assessments?A
Supplier data may or may not be useful to support a dossier. This will depend on the quality of the data and the manner in which those data were acquired (e.g., extraction conditions and analytical methods used). Generally, any supplier data will only support extractable testing because that is more readily generalized than leachable testing with drug product. Sponsors are encouraged to conduct a rigorous assessment of supplier data and experimental design to determine if supplier extractable data are adequately supportive. For example, are the extraction conditions relevant to the intended drug product? Are the analytical methods sensitive enough to detect at the required AET? Are the analytical methods broad enough to survey a broad range of compounds?
-
QFor one-time or infrequently dosed products, can the higher TTC values in M7 be used in lieu of the PQRI- recommended SCT of 0.15 μg/day for OINDP and 1.5 μg/day for injectables?A
As addressed previously, the PQRI-recommended SCT for a given route of administration is intended as a fixed threshold for the identification and reporting of leachables. M7 is best used as a qualification / assessment tool after mutagenic impurities are identified.
-
QIs a leachable study that targets only observed extractables sufficient?A
Extractable testing is performed to gather a chemical understanding of a material, component, or system. From extraction testing, leachable targets may be derived. It is recommended that leachable methods are capable of detecting the targets to be qualified. However, to the extent possible, it is recommended that I eachable studies be broadly capable of detecting other compounds beyond those targeted. For example, sometimes an API will react with a migrating compound, an excipient, or another leachable to create a new related substance. Such a substance would not be seen in a controlled extraction study using only clean solvents lacking API or drug product formulation components. As a result, such a reaction product would not be targeted as a leachable. If the follow-up leachable study only interrogated targets from the extraction study, such a substance would not be detected. Exceptions to this general recommendation may includ e leachables that are not readily detectable or leachables present in drug products formulated from complex matrices such that only targeted leachable analyses (such as LC-MS/MS) are practically capable of detecting the target compounds.
-
QHow do you determine if you have a compound of concern to manage?A
Compounds of concern that may be toxic at very low levels such as N-nitrosamines or polyaromatic hydrocarbons (PAHs) often require highly sensitive and selective analytica I methods in order to be detected. Sponsors should use a risk-based approach driven by the materials used in the packaging system. For example, polyolefins are unlikely to c ontain nitrosamines or PAHs, but these compounds could be found in black rubber stoppers. Often, raw material or component suppliers can provide information about whether their materials contain such compounds of concern. This information can be incorporated into a sponsor's risk analysis.
-
QCan E&L data be leveraged from a generic or platform perspective?A
For a company that fills multiple products into the same or similar packaging components, extractable data can often be generalized to support an entire product line provided that the extraction studies are conducted under conditions supportive of all intended formulations. For instances where the same packaging materials are made available as containers with different volumes, the largest surface area (proportional to the mass of material available to be extracted) to product fill volume ratio is considered worst case. In these examples, a few experiments can support multiple products from an extractables perspective. Leachable studies are more difficult to genericize because the goal is to understand the specific interaction between the formulation and the packaging system. However, there may be opportunities to simplify the number of studies performed across multiple formulations of the same composition where a formulation containing higher concentrations or more excipients/APIs may support an otherwise identical for mulation lacking an ingredient or formulated with lower concentrations of some ingredients. In all cases, genericizing a study to apply to multiple products requires a sound scientific rationale. Agreement with regulators on such a strategy is recommended.