Sterile Filter E&L - EU GMP Annex1
가이드라인 [2019.9]
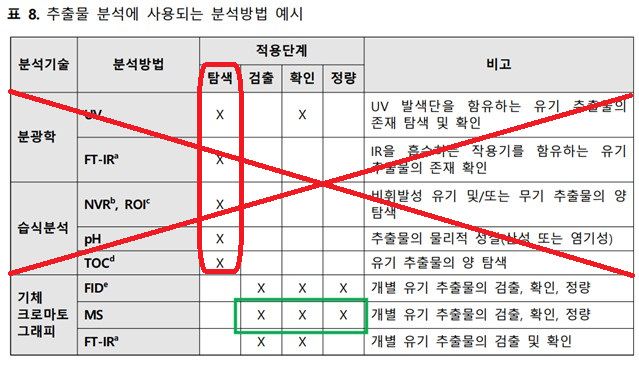
나. 침출물 분석
현재 모든 유기 및 무기화합물을 동시에 정성, 정량할 수 있는 분석 기술이나 분석 기술의 조합은 없다. 침출물에 존재하는 화학물을 여러 보완적인 분석기술을 사용하여 분석하여 침출물 프로파일을 생성하고, 평가한다. 침출물 분석의 목표는 현재 과학기술에 근거한 합리적인 수준에서, 안전성을 고려한 특정 수준 이상으로 의약품에 침출되어 존재하는 물질을 검출, 확인, 정량하는 것이다. 추출물에 대한 위해분석 결과에 따라 침출물 시험 여부를 결정할 수 있다. 침출물 분석을 위한 분석방법은 추출물 특성분석에 적용되는 기술과 동일하므로 이를 참고한다. 다만, 의약품 중 존재하는 첨가제가 탐색을 방해할 수 있기 때문에 일반적으로 탐색 분석은 침출물 분석에 사용하지 않는다.
EU GMP Annex 1
The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use
Annex 1
Manufacture of Sterile Medicinal Products
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Single use systems (SUS)
8.131 SUS are those technologies used in manufacture of sterile products which are used as an alternative to reusable equipment. SUS can be individual components or made up of multiple components such as bags, filters, tubing, connectors, valves, storage bottles and sensors. Single use systems should be designed to reduce the need for manipulations and complexity of manual interventions.
8.132 There are some specific risks associated with SUS which should be assessed as part of the CCS. These risks include but are not limited to:
i. The interaction between the product and product contact surface (such as adsorption, or leachables and extractables).
ii. The fragile nature of the system compared with fixed reusable systems.
iii. The increase in the number and complexity of manual operations (including inspection and handling of the system) and connections made.
iv. The complexity of the assembly.
v. The performance of the pre- and post-use integrity testing for sterilising grade filters (see paragraph 8.87).
vi. The risk of holes and leakage.
vii. The potential for compromising the system at the point of opening the outer packaging.
viii. The risk of particle contamination.
~~~~~
8.136 The extractable and leachable profiles of the SUS and any impact on the quality of the product especially where the system is made from polymer-based materials should be evaluated. An assessment should be carried out for each component to evaluate the applicability of the extractable profile data. For components considered to be at high risk from leachables, including those that may absorb processed materials or those with extended material contact times, an assessment of leachable profile studies, including safety concerns, should be taken into consideration. If applying simulated processing conditions, these should accurately reflect the actual processing conditions and be based on a scientific rationale.

개인정보처리방침
주식회사 제이앤케이는 개인정보보호법에 따라 정보주체의 개인정보를 보호하고 이와 관련한 고충을 신속하고 원활하게 처리할 수 있도록 하기 위하여 다음과 같이 개인정보 처리지침을 수립·공개합니다.
제1조(개인정보의 처리 목적)
주식회사 제이앤케이는 개인정보를 다음의 목적을 위해 처리합니다. 처리한 개인정보는 다음의 목적이외의 용도로는 사용되지 않으며 이용 목적이 변경될 시에는 사전 동의를 구할 예정입니다.
가. 재화 또는 서비스 제공상담서비스 제공
콘텐츠 제공 등을 목적으로 개인정보를 처리합니다.
제2조(개인정보의 처리 및 보유기간)
주식회사 제이앤케이는 법령에 따른 개인정보 보유·이용기간 또는 정보주체로부터 개인정보를 수집 시에 동의 받은 개인정보 보유·이용기간 내에서 개인정보를 처리·보유합니다.
1. 사업신청: 연 사업종료 시
2. 자원봉사 신청 : 연 사업종료 시
제3조(개인정보의 제3자 제공)
주식회사 제이앤케이는 정보주체의 동의, 법률의 특별한 규정 등 개인정보 보호법 제 17조 및 제18조에 해당하는 경우에만 개인정보를 제3자에게 제공합니다.
제4조(개인정보처리의 위탁)
주식회사 제이앤케이는 원활한 개인정보 업무처리를 위하여 다음과 같이 개인정보 처리업무를 위탁하고 있습니다.
- 위탁받는 자 (수탁자) : 솔루웨이
- 위탁하는 업무의 내용 : 홈페이지 시스템 운영 및 유지보수
제5조(정보주체와 법정대리인의 권리·의무 및 행사방법)
이용자는 개인정보주체로서 다음과 같은 권리를 행사할 수 있습니다.
1. 정보주체는 주식회사 제이앤케이에 대해 언제든지 다음 각 호의 개인정보 보호 관련 권리를 행사할 수 있습니다.
(1) 개인정보 열람요구
(2) 오류 등이 있을 경우 정정 요구
(3) 삭제요구
(4) 처리정지 요구
2. 제1항에 따른 권리 행사는 주식회사 제이앤케이에 대해 개인정보 보호법 시행령 제41조 제1항에 따라 서면, 전자우편, 모사전송(FAX) 등을 통하여 하실 수 있으며 주식회사 제이앤케이는 이에 대해 지체 없이 조치하겠습니다.
3. 정보주체가 개인정보의 오류 등에 대한 정정 또는 삭제를 요구한 경우에는 주식회사 제이앤케이는 정정 또는 삭제를 완료할 때까지 당해 개인정보를 이용하거나 제공하지 않습니다.
4. 제1항에 따른 권리 행사는 정보주체의 법정대리인이나 위임을 받은 자 등 대리인을 통하여 하실 수 있습니다. 이 경우 개인정보 보호법 시행규칙 별지 제11호 서식에 따른 위임장을 제출하셔야 합니다.
5. 개인정보 열람 및 처리정지 요구는 개인정보보호법 제35조 제5항, 제37조 제2항에 의하여 정보주체의 권리가 제한될 수 있습니다.
6. 개인정보의 정정 및 삭제 요구는 다른 법령에서 그 개인정보가 수집 대상으로 명시되어 있는 경우에는 그 삭제를 요구할 수 없습니다.
7. 주식회사 제이앤케이는 정보주체 권리에 따른 열람의 요구, 정정·삭제의 요구, 처리정지의 요구 시 열람 등 요구를 한 자가 본인이거나 정당한 대리인인지를 확인합니다.
제6조(처리하는 개인정보 항목)
주식회사 제이앤케이는 다음의 개인정보 항목을 처리하고 있습니다.
재화 및 서비스제공
1. 사업신청: 기관/단체명, 기관주소, 이름 및 직책, 전화번호(사무실, 휴대전화), 전자우편 주소
2. 자원봉사 신청: 이름, 나이, 전화번호, 소속(학교 및 기관), 전자우편 주소, 경력사항
3. 인터넷 서비스 이용과정에서 아래 개인정보 항목이 자동으로 생성되어 수집될 수 있습니다. IP주소, 쿠키, MAC주소, 서비스이용기록, 방문기록, 불량이용기록 등
제7조(개인정보의 파기)
주식회사 제이앤케이는 다음의 개인정보 항목을 처리하고 있습니다.
재화 및 서비스제공
1. 주식회사 제이앤케이는 원칙적으로 개인정보 처리목적 달성 등 개인정보가 불필요하게 되었을 때에는 지체 없이 해당 개인정보를 파기합니다.
2. 정보주체로부터 동의 받은 개인정보 보유기간이 경과하거나 처리목적이 달성되었음에도 불구하고 다른 법령에 따라 개인정보를 계속 보존하여야 하는 경우에는, 해당 개인정보를 별도의 데이터베이스(DB)로 옮기거나 보관 장소를 달리하여 보존합니다.
3. 개인정보 파기의 절차 및 방법은 다음과 같습니다.
(1) 파기절차: 주식회사 제이앤케이는 파기하여야 하는 개인정보에 대해 개인정보 파기계획을 수립하여 파기합니다. 주식회사 제이앤케이는 파기사유가 발생한 개인정보를 선정하고, 주식회사 제이앤케이의 개인정보 보호책임자의 승인을 받아 개인정보를 파기합니다.
(2) 파기방법: 주식회사 제이앤케이는 전자적 파일 형태의 정보는 기록을 재생할 수 없도록 파기하며, 종이 문서에 기록·저장된 개인정보는 분쇄기로 분쇄하거나 소각하여 파기합니다.
제8조(개인정보의 안전성 확보조치)
주식회사 제이앤케이는 개인정보의 안전성 확보를 위해 다음과 같은 조치를 취하고 있습니다.
1. 관리적 조치 : 내부관리계획 수립·시행, 정기적 직원 교육 등
2. 기술적 조치 : 개인정보처리시스템 등의 접근권한 관리, 개인정보 접속기록관리시스템 운영, 고유식별정보 등의 암호화, 보안프로그램 설치
3. 물리적 조치 : 자료보관실 등의 접근통제
제9조(개인정보 자동 수집 장치의 설치·운영 및 거부에 관한 사항)
주식회사 제이앤케이는 개인정보보호법 제29조에 따라 다음과 같이 안전성 확보에 필요한 기술적/관리적 및 물리적 조치를 하고 있습니다.
1. 주식회사 제이앤케이는 이용자에게 개별적인 맞춤서비스를 제공하기 위해 이용정보를 저장하고 수시로 불러오는 '쿠키(cookie)'를 사용합니다.
2. 쿠키는 웹사이트를 운영하는데 이용되는 서버(http)가 이용자의 컴퓨터 브라우저에게 보내는 소량의 정보이며 이용자의 PC컴퓨터 내의 하드디스크에 저장되기도 합니다.
1) 쿠키의 사용목적: 이용자가 방문한 각 서비스와 웹 사이트들에 대한 방문 및 이용형태, 인기검색어, 보안접속 여부 등을 파악하여 이용자에게 최적화된 정보제공을 위해 사용됩니다.
2) 쿠키의 설치·운영 및 거부: 웹브라우저 상단의 도구→인터넷옵션→개인정보 메뉴의 옵션 설정을 통해 쿠키 저장을 거부할 수 있습니다.
3) 쿠키 저장을 거부할 경우 맞춤형 서비스 이용에 어려움이 발생할 수 있습니다.
제10조(개인정보 보호책임자)
1. 주식회사 제이앤케이는 개인정보 처리에 관한 업무를 총괄해서 책임지고, 개인정보 처리와 관련한 정보주체의 불만처리 및 피해구제 등을 위하여 아래와 같이 개인정보 보호책임자를 지정하고 있습니다.
▶ 개인정보 보호책임자: ***
- e-mail: jnkkorea@jnkkorea.co.kr
- 전화번호: +82-43-234-0367
2. 정보주체께서는 주식회사 제이앤케이의 서비스(또는 사업)를 이용하시면서 발생한 모든 개인정보 보호 관련 문의, 불만처리, 피해구제 등에 관한 사항을 개인정보 보호책임자 및 담당부서로 문의하실 수 있습니다. 주식회사 제이앤케이는 정보주체의 문의에 대해 지체 없이 답변 및 처리해드릴 것입니다.
제11조(개인정보 열람청구)
정보주체는 개인정보 보호법 제35조에 따른 개인정보의 열람 청구를 개인정보 보호 담당부서로 할 수 있습니다. 주식회사 제이앤케이는 정보주체의 개인정보 열람청구가 신속하게 처리되도록 노력하겠습니다.
정보주체께서는 제1항의 열람청구 접수·처리부서 이외에, 행정안전부의 ‘개인정보보호 종합지원 포털’ 웹사이트(www.privacy.go.kr)를 통하여서도 개인정보 열람청구를 하실 수 있습니다.
▶ 행정안전부 개인정보보호 종합지원 포털 → 개인정보 민원 → 개인정보 열람등 요구 (공공아이핀을 통한 실명인증 필요)
제12조(권익침해 구제방법)
정보주체는 아래의 기관에 대해 개인정보 침해에 대한 피해구제, 상담 등을 문의하실 수 있습니다.
※ 아래의 기관은 주식회사 제이앤케이와는 별개의 기관으로서, 주식회사 제이앤케이의 자체적인 개인정보 불만처리, 피해구제 결과에 만족하지 못하시거나 보다 자세한 도움이 필요하시면 문의하여 주시기 바랍니다.
□ 개인정보 침해신고센터 (한국인터넷진흥원 운영)
- 소관업무 : 개인정보 침해사실 신고, 상담 신청
- 홈페이지 : privacy.kisa.or.kr
- 전화 : (국번없이) 118
- 주소 : (05717) 서울특별시 송파구 중대로 135 한국인터넷진흥원 개인정보침해신고센터
□ 개인정보 분쟁조정위원회
- 소관업무 : 개인정보 분쟁조정신청, 집단분쟁조정 (민사적 해결)
- 홈페이지 : www.kopico.go.kr
- 전화 : (국번없이) 1833-6972
- 주소 : (03171) 서울특별시 종로구 세종대로 209 정부서울청사 4층
□ 검찰청 사이버범죄수사단
- 소관업무 : 개인정보 침해 관련 형사사건 문의 및 신고
- 홈페이지 : www.spo.go.kr
- 전화 : 02-3480-3573
□ 검찰청 사이버안전국
- 소관업무 : 개인정보 사이버범죄 신고 및 상담고
- 홈페이지 : cyberbureau.police.go.kr
- 전화 : (국번없이) 182
제13조(영상정보처리 설치·운영)
주식회사 제이앤케이는 영상정보처리기기를 설치·운영하고 있지 않습니다.
제14조(개인정보 처리방침 변경)
이 개인정보 처리방침은 2023. 01. 31 적용됩니다.

나. 침출물 분석
현재 모든 유기 및 무기화합물을 동시에 정성, 정량할 수 있는 분석 기술이나 분석 기술의 조합은 없다. 침출물에 존재하는 화학물을 여러 보완적인 분석기술을 사용하여 분석하여 침출물 프로파일을 생성하고, 평가한다. 침출물 분석의 목표는 현재 과학기술에 근거한 합리적인 수준에서, 안전성을 고려한 특정 수준 이상으로 의약품에 침출되어 존재하는 물질을 검출, 확인, 정량하는 것이다. 추출물에 대한 위해분석 결과에 따라 침출물 시험 여부를 결정할 수 있다. 침출물 분석을 위한 분석방법은 추출물 특성분석에 적용되는 기술과 동일하므로 이를 참고한다. 다만, 의약품 중 존재하는 첨가제가 탐색을 방해할 수 있기 때문에 일반적으로 탐색 분석은 침출물 분석에 사용하지 않는다.
EU GMP Annex 1
The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use
Annex 1
Manufacture of Sterile Medicinal Products
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Single use systems (SUS)
8.131 SUS are those technologies used in manufacture of sterile products which are used as an alternative to reusable equipment. SUS can be individual components or made up of multiple components such as bags, filters, tubing, connectors, valves, storage bottles and sensors. Single use systems should be designed to reduce the need for manipulations and complexity of manual interventions.
8.132 There are some specific risks associated with SUS which should be assessed as part of the CCS. These risks include but are not limited to:
i. The interaction between the product and product contact surface (such as adsorption, or leachables and extractables).
ii. The fragile nature of the system compared with fixed reusable systems.
iii. The increase in the number and complexity of manual operations (including inspection and handling of the system) and connections made.
iv. The complexity of the assembly.
v. The performance of the pre- and post-use integrity testing for sterilising grade filters (see paragraph 8.87).
vi. The risk of holes and leakage.
vii. The potential for compromising the system at the point of opening the outer packaging.
viii. The risk of particle contamination.
~~~~~
8.136 The extractable and leachable profiles of the SUS and any impact on the quality of the product especially where the system is made from polymer-based materials should be evaluated. An assessment should be carried out for each component to evaluate the applicability of the extractable profile data. For components considered to be at high risk from leachables, including those that may absorb processed materials or those with extended material contact times, an assessment of leachable profile studies, including safety concerns, should be taken into consideration. If applying simulated processing conditions, these should accurately reflect the actual processing conditions and be based on a scientific rationale.

개인정보처리방침
주식회사 제이앤케이는 개인정보보호법에 따라 정보주체의 개인정보를 보호하고 이와 관련한 고충을 신속하고 원활하게 처리할 수 있도록 하기 위하여 다음과 같이 개인정보 처리지침을 수립·공개합니다.
제1조(개인정보의 처리 목적)
주식회사 제이앤케이는 개인정보를 다음의 목적을 위해 처리합니다. 처리한 개인정보는 다음의 목적이외의 용도로는 사용되지 않으며 이용 목적이 변경될 시에는 사전 동의를 구할 예정입니다.
가. 재화 또는 서비스 제공상담서비스 제공
콘텐츠 제공 등을 목적으로 개인정보를 처리합니다.
제2조(개인정보의 처리 및 보유기간)
주식회사 제이앤케이는 법령에 따른 개인정보 보유·이용기간 또는 정보주체로부터 개인정보를 수집 시에 동의 받은 개인정보 보유·이용기간 내에서 개인정보를 처리·보유합니다.
1. 사업신청: 연 사업종료 시
2. 자원봉사 신청 : 연 사업종료 시
제3조(개인정보의 제3자 제공)
주식회사 제이앤케이는 정보주체의 동의, 법률의 특별한 규정 등 개인정보 보호법 제 17조 및 제18조에 해당하는 경우에만 개인정보를 제3자에게 제공합니다.
제4조(개인정보처리의 위탁)
주식회사 제이앤케이는 원활한 개인정보 업무처리를 위하여 다음과 같이 개인정보 처리업무를 위탁하고 있습니다.
- 위탁받는 자 (수탁자) : 솔루웨이
- 위탁하는 업무의 내용 : 홈페이지 시스템 운영 및 유지보수
제5조(정보주체와 법정대리인의 권리·의무 및 행사방법)
이용자는 개인정보주체로서 다음과 같은 권리를 행사할 수 있습니다.
1. 정보주체는 주식회사 제이앤케이에 대해 언제든지 다음 각 호의 개인정보 보호 관련 권리를 행사할 수 있습니다.
(1) 개인정보 열람요구
(2) 오류 등이 있을 경우 정정 요구
(3) 삭제요구
(4) 처리정지 요구
2. 제1항에 따른 권리 행사는 주식회사 제이앤케이에 대해 개인정보 보호법 시행령 제41조 제1항에 따라 서면, 전자우편, 모사전송(FAX) 등을 통하여 하실 수 있으며 주식회사 제이앤케이는 이에 대해 지체 없이 조치하겠습니다.
3. 정보주체가 개인정보의 오류 등에 대한 정정 또는 삭제를 요구한 경우에는 주식회사 제이앤케이는 정정 또는 삭제를 완료할 때까지 당해 개인정보를 이용하거나 제공하지 않습니다.
4. 제1항에 따른 권리 행사는 정보주체의 법정대리인이나 위임을 받은 자 등 대리인을 통하여 하실 수 있습니다. 이 경우 개인정보 보호법 시행규칙 별지 제11호 서식에 따른 위임장을 제출하셔야 합니다.
5. 개인정보 열람 및 처리정지 요구는 개인정보보호법 제35조 제5항, 제37조 제2항에 의하여 정보주체의 권리가 제한될 수 있습니다.
6. 개인정보의 정정 및 삭제 요구는 다른 법령에서 그 개인정보가 수집 대상으로 명시되어 있는 경우에는 그 삭제를 요구할 수 없습니다.
7. 주식회사 제이앤케이는 정보주체 권리에 따른 열람의 요구, 정정·삭제의 요구, 처리정지의 요구 시 열람 등 요구를 한 자가 본인이거나 정당한 대리인인지를 확인합니다.
제6조(처리하는 개인정보 항목)
주식회사 제이앤케이는 다음의 개인정보 항목을 처리하고 있습니다.
재화 및 서비스제공
1. 사업신청: 기관/단체명, 기관주소, 이름 및 직책, 전화번호(사무실, 휴대전화), 전자우편 주소
2. 자원봉사 신청: 이름, 나이, 전화번호, 소속(학교 및 기관), 전자우편 주소, 경력사항
3. 인터넷 서비스 이용과정에서 아래 개인정보 항목이 자동으로 생성되어 수집될 수 있습니다. IP주소, 쿠키, MAC주소, 서비스이용기록, 방문기록, 불량이용기록 등
제7조(개인정보의 파기)
주식회사 제이앤케이는 다음의 개인정보 항목을 처리하고 있습니다.
재화 및 서비스제공
1. 주식회사 제이앤케이는 원칙적으로 개인정보 처리목적 달성 등 개인정보가 불필요하게 되었을 때에는 지체 없이 해당 개인정보를 파기합니다.
2. 정보주체로부터 동의 받은 개인정보 보유기간이 경과하거나 처리목적이 달성되었음에도 불구하고 다른 법령에 따라 개인정보를 계속 보존하여야 하는 경우에는, 해당 개인정보를 별도의 데이터베이스(DB)로 옮기거나 보관 장소를 달리하여 보존합니다.
3. 개인정보 파기의 절차 및 방법은 다음과 같습니다.
(1) 파기절차: 주식회사 제이앤케이는 파기하여야 하는 개인정보에 대해 개인정보 파기계획을 수립하여 파기합니다. 주식회사 제이앤케이는 파기사유가 발생한 개인정보를 선정하고, 주식회사 제이앤케이의 개인정보 보호책임자의 승인을 받아 개인정보를 파기합니다.
(2) 파기방법: 주식회사 제이앤케이는 전자적 파일 형태의 정보는 기록을 재생할 수 없도록 파기하며, 종이 문서에 기록·저장된 개인정보는 분쇄기로 분쇄하거나 소각하여 파기합니다.
제8조(개인정보의 안전성 확보조치)
주식회사 제이앤케이는 개인정보의 안전성 확보를 위해 다음과 같은 조치를 취하고 있습니다.
1. 관리적 조치 : 내부관리계획 수립·시행, 정기적 직원 교육 등
2. 기술적 조치 : 개인정보처리시스템 등의 접근권한 관리, 개인정보 접속기록관리시스템 운영, 고유식별정보 등의 암호화, 보안프로그램 설치
3. 물리적 조치 : 자료보관실 등의 접근통제
제9조(개인정보 자동 수집 장치의 설치·운영 및 거부에 관한 사항)
주식회사 제이앤케이는 개인정보보호법 제29조에 따라 다음과 같이 안전성 확보에 필요한 기술적/관리적 및 물리적 조치를 하고 있습니다.
1. 주식회사 제이앤케이는 이용자에게 개별적인 맞춤서비스를 제공하기 위해 이용정보를 저장하고 수시로 불러오는 '쿠키(cookie)'를 사용합니다.
2. 쿠키는 웹사이트를 운영하는데 이용되는 서버(http)가 이용자의 컴퓨터 브라우저에게 보내는 소량의 정보이며 이용자의 PC컴퓨터 내의 하드디스크에 저장되기도 합니다.
1) 쿠키의 사용목적: 이용자가 방문한 각 서비스와 웹 사이트들에 대한 방문 및 이용형태, 인기검색어, 보안접속 여부 등을 파악하여 이용자에게 최적화된 정보제공을 위해 사용됩니다.
2) 쿠키의 설치·운영 및 거부: 웹브라우저 상단의 도구→인터넷옵션→개인정보 메뉴의 옵션 설정을 통해 쿠키 저장을 거부할 수 있습니다.
3) 쿠키 저장을 거부할 경우 맞춤형 서비스 이용에 어려움이 발생할 수 있습니다.
제10조(개인정보 보호책임자)
1. 주식회사 제이앤케이는 개인정보 처리에 관한 업무를 총괄해서 책임지고, 개인정보 처리와 관련한 정보주체의 불만처리 및 피해구제 등을 위하여 아래와 같이 개인정보 보호책임자를 지정하고 있습니다.
▶ 개인정보 보호책임자: ***
- e-mail: jnkkorea@jnkkorea.co.kr
- 전화번호: +82-43-234-0367
2. 정보주체께서는 주식회사 제이앤케이의 서비스(또는 사업)를 이용하시면서 발생한 모든 개인정보 보호 관련 문의, 불만처리, 피해구제 등에 관한 사항을 개인정보 보호책임자 및 담당부서로 문의하실 수 있습니다. 주식회사 제이앤케이는 정보주체의 문의에 대해 지체 없이 답변 및 처리해드릴 것입니다.
제11조(개인정보 열람청구)
정보주체는 개인정보 보호법 제35조에 따른 개인정보의 열람 청구를 개인정보 보호 담당부서로 할 수 있습니다. 주식회사 제이앤케이는 정보주체의 개인정보 열람청구가 신속하게 처리되도록 노력하겠습니다.
정보주체께서는 제1항의 열람청구 접수·처리부서 이외에, 행정안전부의 ‘개인정보보호 종합지원 포털’ 웹사이트(www.privacy.go.kr)를 통하여서도 개인정보 열람청구를 하실 수 있습니다.
▶ 행정안전부 개인정보보호 종합지원 포털 → 개인정보 민원 → 개인정보 열람등 요구 (공공아이핀을 통한 실명인증 필요)
제12조(권익침해 구제방법)
정보주체는 아래의 기관에 대해 개인정보 침해에 대한 피해구제, 상담 등을 문의하실 수 있습니다.
※ 아래의 기관은 주식회사 제이앤케이와는 별개의 기관으로서, 주식회사 제이앤케이의 자체적인 개인정보 불만처리, 피해구제 결과에 만족하지 못하시거나 보다 자세한 도움이 필요하시면 문의하여 주시기 바랍니다.
□ 개인정보 침해신고센터 (한국인터넷진흥원 운영)
- 소관업무 : 개인정보 침해사실 신고, 상담 신청
- 홈페이지 : privacy.kisa.or.kr
- 전화 : (국번없이) 118
- 주소 : (05717) 서울특별시 송파구 중대로 135 한국인터넷진흥원 개인정보침해신고센터
□ 개인정보 분쟁조정위원회
- 소관업무 : 개인정보 분쟁조정신청, 집단분쟁조정 (민사적 해결)
- 홈페이지 : www.kopico.go.kr
- 전화 : (국번없이) 1833-6972
- 주소 : (03171) 서울특별시 종로구 세종대로 209 정부서울청사 4층
□ 검찰청 사이버범죄수사단
- 소관업무 : 개인정보 침해 관련 형사사건 문의 및 신고
- 홈페이지 : www.spo.go.kr
- 전화 : 02-3480-3573
□ 검찰청 사이버안전국
- 소관업무 : 개인정보 사이버범죄 신고 및 상담고
- 홈페이지 : cyberbureau.police.go.kr
- 전화 : (국번없이) 182
제13조(영상정보처리 설치·운영)
주식회사 제이앤케이는 영상정보처리기기를 설치·운영하고 있지 않습니다.
제14조(개인정보 처리방침 변경)
이 개인정보 처리방침은 2023. 01. 31 적용됩니다.