C-Flex® Tubing for Biopharmaceutical Applications
C-Flex® is the original patented thermoplastic elastomer tubing specifically designed to meet the critical demands of pharmaceutical and biopharmaceutical applications for fluid processing with excellent heat seal and sterile weld capabilities. For over 25 years, C-Flex has been the thermoplastic elastomer tubing most widely used and validated by the world's leading pharmaceutical and biopharmaceutical companies. Each coil of C-Flex tubing is extruded to precise ID and wall dimensions. All tubing is formulated to meet the standards of the biopharmaceutical industry and is quality tested before leaving the Saint-Gobain Performance Plastics production facilities.
Applications
Aseptic sealing disconnections
Aseptic welding connections
Ideal for use in single-use assemblies
Buffer and media preparation
Cell culture operations
Purification operations
Diagnostic products
Biopharma manufacturing
Single-use fluid transfer sets
Tubing and bags manifolds
Laboratory R&D
High-purity water systems
The Material Difference
C-Flex tubing is part of a family of TPE tubing offering the widest range of formulations and sizes in the industry including translucent tubing for fluid flow visibility, opaque tubing for application when sensitivity for light is required and custom formulations from 50A to 80A durometer. C-Flex is also available as C-Flex Braided to use in applications where a thermoplastic tube is desired, but the pressure requirements exceed those allowable by a non-reinforced thermoplastic material. All are available in custom sizes, lengths and packaging. C-Flex tubing has a secure global supply chain with redundant manufacturing sites in the United States and Europe with validated manufacturing processes.
Easy Validation Process
C-Flex tubing is manufactured from pharmaceutical-grade thermoplastic materials and is fully characterized, validated and tested to a variety of specifications including USP Class VI, ISO 10993-3 (Ames Genotoxicity), ISO 10993-4 (Hemolysis, Indirect), ISO 10993-5 (Cytotoxicity, In-Vitro), ISO 10993-11 (Systemic Toxicity, In-Vivo) and European Pharmacopeia. Extensive biological, chemical, physical properties and extractable testing performed on unsterilized and sterilized C-Flex tubing is available to assist in the validation of C-Flex application.
Features / Benefits
Sealable and weldable either pre- or post-sterilization
Sterilizable by gamma irradiation and autoclave
Validation package available for all C-Flex® commercial formulations
Moldable, bondable and formable for single-use assemblies and overmolds
Non-pyrogenic, non-cytotoxic, non-hemolytic
Remains flexible from -45ºC to 135ºC (-50ºF to 275ºF)
Significantly less permeable than silicone
Smooth inner bore for superior fluid flow
All formulas are Animal-Derived Component Free
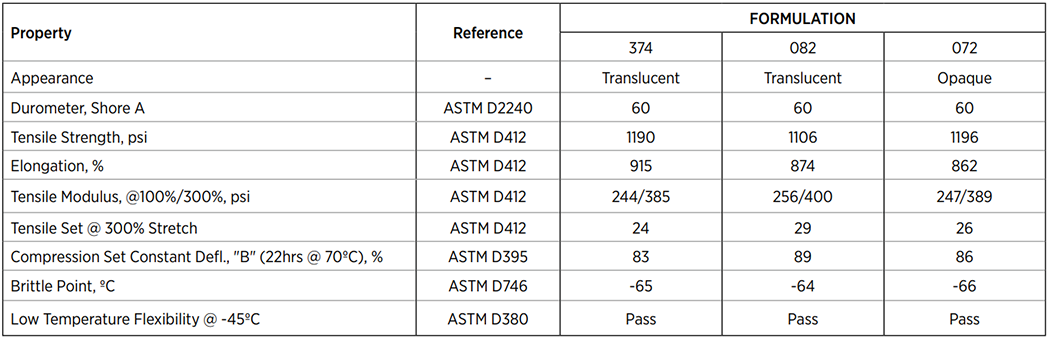
Typical Physical Properties of C-Flex®
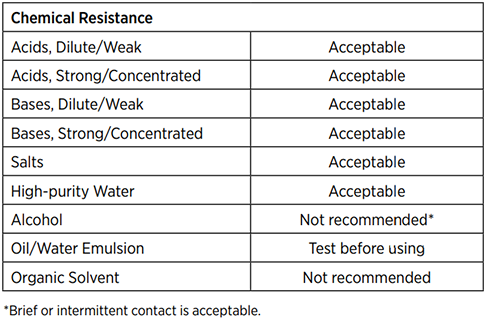
NOTE: It is the users responsibility to insure the suitability and safety of C-Flex for all intended uses/applications.
Sterilization Methods
Autoclavable - one time 30 minute cycle at 121ºC
Radiation - up to 40kGy
NOTE: C-Flex tubing will deteriorate with repeated autoclaving. Radiation is the recommended method of sterilization for all C-Flex thermoplastic materials.
Characteristics
The manufacturing process is carefully controlled from raw material through production. Inspection and lot traceability information is readily accessible as batch numbers are assigned. All packages are identified by external labeling on both the bag and the crush-proof box.
-
C-Flex comes in a wide variety of sizes and formulations.
-

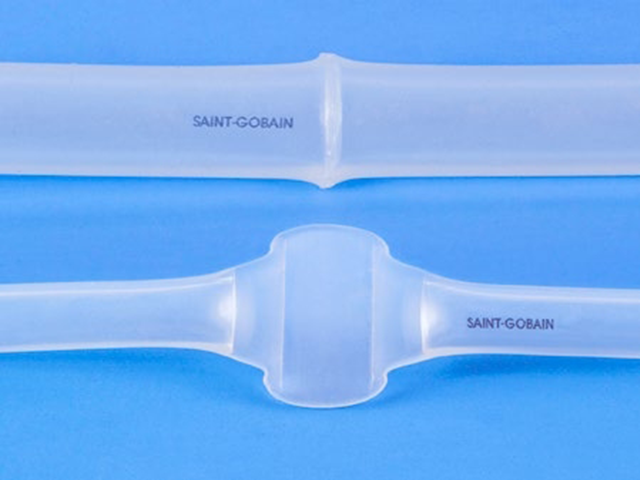
One-piece overmolded C-Flex manifold maintains inner bore diameters with superior reliability and product integrity.
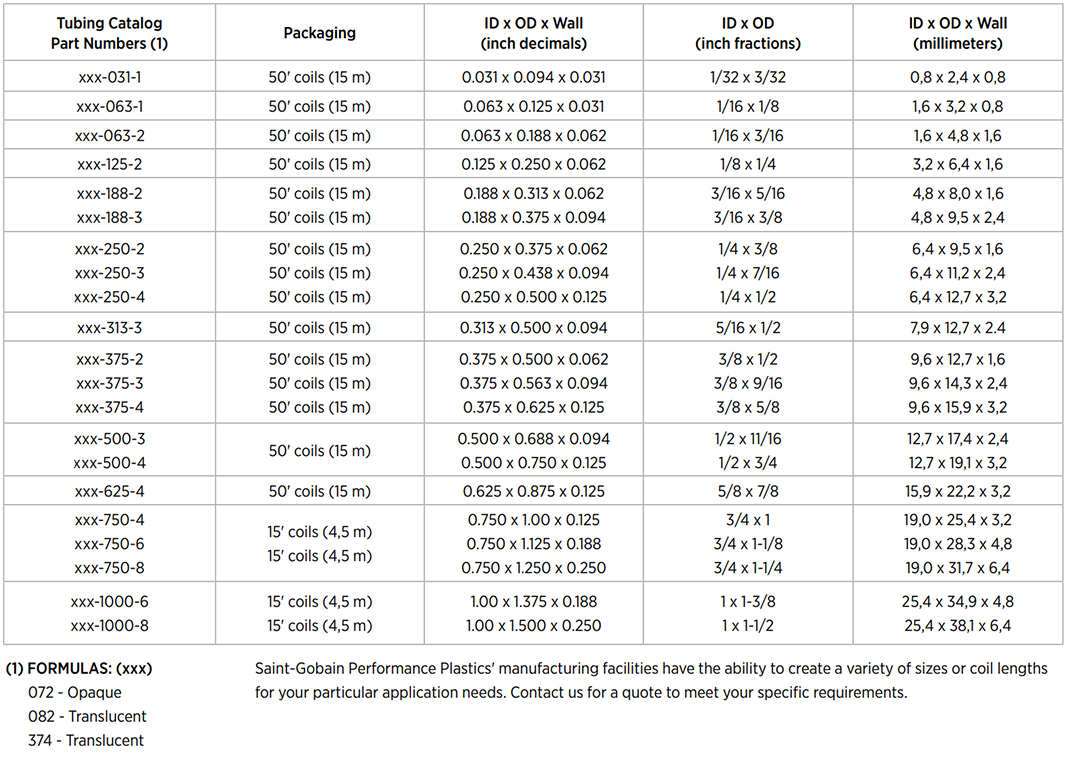
C-Flex® Tubing Selection Guide